Home
 |
About UsWelcome to the Markelz Lab! |
|
|
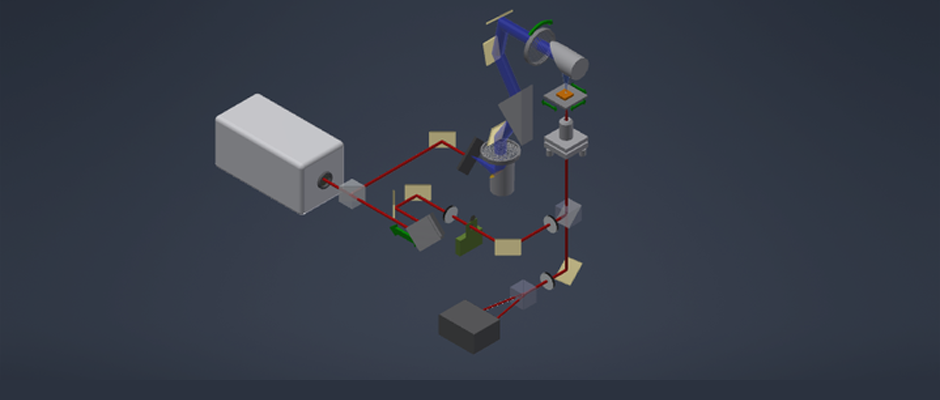
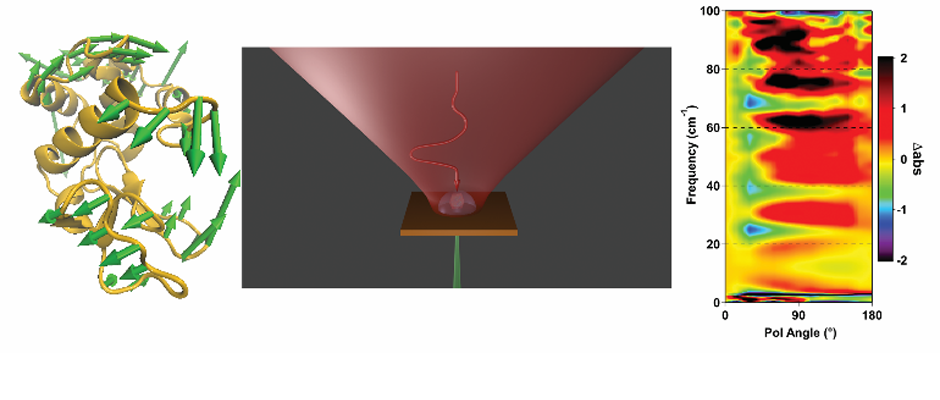
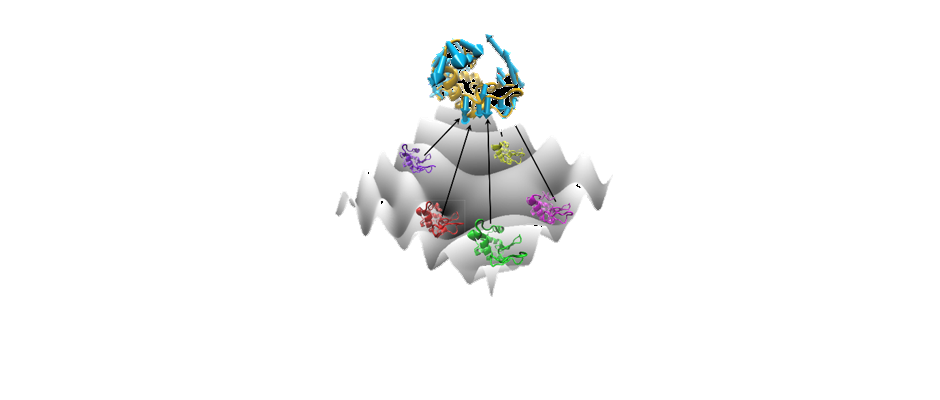
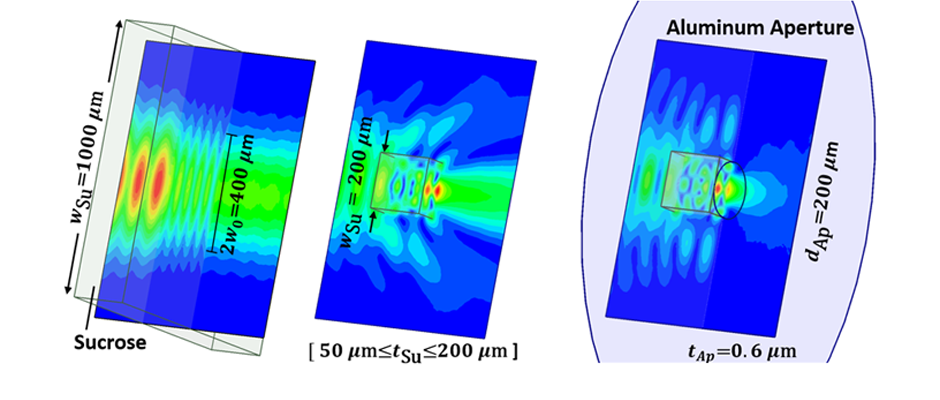
We are studying biomolecular dynamics and collective excitations in optical and electronic materials using a variety of tools. The overlapping technology for these diverse topics is terahertz spectroscopy. In particular we use terahertz time-domain spectroscopy (THz-TDS) to measure protein intramolecular vibrations, solvent dielectric response, crystal phonons, and electronic excitations in novel materials. Terahertz light is uniquely suited to the study of these systems. In addition to the experimental work, we use molecular dynamics simulations to explore the role of structural dynamics in protein function.
Activities over the years have been supported by the National Science Foundation, the American Chemical Society, the Office of Army Research, Department of Energy, NIH, and the Research Corporation. |